A Cardiff-based company with a technology to manage smoke created by surgical tools in operating theatres has raised £5 million for growth.
The latest funding brings the total raised by Alesi Surgical to date to over £21 million. It comes as Alesi secures FDA clearance in the US for the IonPencil, the first surgical tool to incorporate its technology for use in routine surgery, which represents approximately 80% of all surgical procedures.
A spin-out from Cardiff University, Alesi is based at the Cardiff Medicentre and currently has a 15-strong team.
The hazards of smoke generated by electronic surgical tools are increasingly being recognised. Smoke reduces visibility and fogs cameras during keyhole surgery, interrupting workflow. It also creates health risks for theatre staff due to the presence of toxins and viruses in human tissue.
Legislation has already been passed in 18 US states requiring a smoke management policy in all surgical procedures, with more expected to follow. However current techniques, such as extraction tubes which must be attached to surgical instruments, are cumbersome, noisy and unpopular with surgeons.
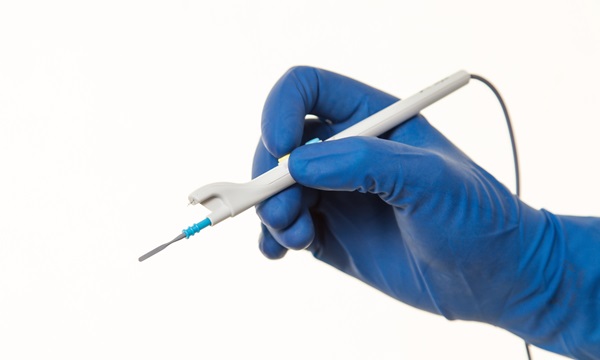
Alesi’s system uses electrical filtration to remove surgical smoke from the atmosphere. Its first-generation Ultravision system was designed for laparoscopic surgery and has been used in over 40,000 procedures. Independent research has shown that it is 23 times better than alternative solutions at minimising smoke release during laparoscopies and that it captures and reduces the infectivity of viruses in the smoke.
The latest version, Ultravision2, which is FDA approved, is a platform technology that can be integrated into surgical tools and existing theatre equipment. Its first integrated tool, for use in laparoscopic surgery, already has FDA approval and the launch of the IonPencil for routine surgery means it now offers smoke management solutions for all surgical procedures that create smoke. There are over 40 million such procedures performed in the USA, Europe, and Japan every year.
The funding round was led by Mercia Ventures and included existing investors IP Group and Panakès Partners. It will enable the firm to drive sales in the US market and to seek regulatory approvals for its products in Europe and Japan.
Dominic Griffiths, CEO of Alesi Surgical, said:
“We have been overwhelmed with the positive feedback we received from surgeons during the development of the IonPencil. As they have been reluctant to adopt the current systems, we believe our device will greatly improve compliance with new legislation. We are also pleased to welcome Mercia Ventures as a new investor alongside our existing investors and are eager to put this latest capital to good use to fuel our future growth.”
Robert Hornby, Investor Director at Mercia Ventures, added:
“The introduction of new regulations will drive uptake of smoke management solutions in the years ahead. Alesi’s technology offers many advantages over current systems. The company has already established a name for itself with its laparoscopy systems but the introduction of the IonPencil will open up a much wider market. We look forward to supporting Dominic and the team in this new chapter in the company’s growth.”
